IMPROVEMENT OF ACETIC ACID AND FURFURAL PRODUCTION IN A WOOD BIOREFINERY
In view of global warming and volatile prices for fossil resources, the shift to a bio-based industry is of utmost importance. The biorefinery concept aims to convert biomass as completely as possible into energy, fuels, chemicals, and materials. Lignocellulosic feedstock biorefineries use raw materials such as wood or agricultural waste that do not compete with the food and feed industry.
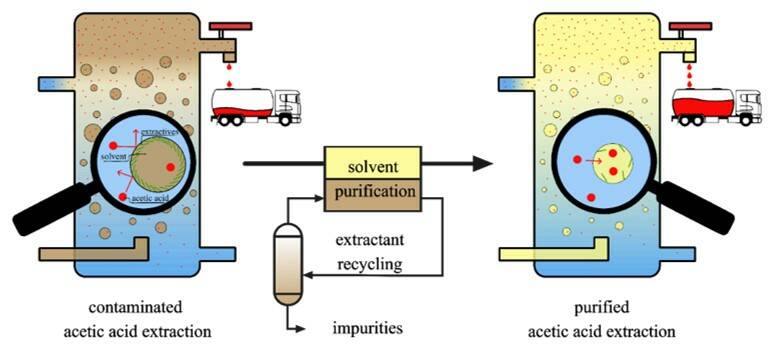
In conjunction with dissolving pulp production, Lenzing AG has been operating an industrial biorefinery plant for the production of acetic acid and furfural for almost 40 years. In the course of sulphite pulping, acetic acid and furfural are formed from the hemicellulose fraction of hardwood. During the chemical recovery, they are then transferred to an aqueous side-stream, the so-called vapour condensate. Lenzing’s biorefinery plant uses a special organic solvent to extract acetic acid and furfural out of this side-stream, after which they are separated and purified. Acetic acid is mainly used in the food industry and furfural serves as an intermediate for a variety of specialty chemicals. However, the biorefinery solvent is heavily contaminated with impurities, leading to problems such as yield losses, emulsion formation and incrustation on plant equipment. So far, the chemical nature of these impurities and their influence on the acetic acid and furfural production was unknown.
Contaminations decrease the plant efficiency
Within the COMET project the biorefinery solvent was thoroughly analysed with different methods. Two major classes of impurities were identified: furfural degradation products & wood extractives. Experiments revealed that furfural forms heterogeneous polymeric structures during degradation at acidic conditions and high temperatures. These polymers cause discoloration of the solvent and the formation of solid particles that incrust equipment parts. Consequently, periodic cleaning shutdowns are necessary. High levels of wood extractives, consisting mainly of fatty acids and resin acids, were found to accumulate in the biorefinery solvent. Fatty acids make up the lipids of hardwood and resin acids protect wood against herbivore or pathogen attack.
To clarify the influence of wood extractives on the extraction of acetic acid, experiments with model solutions were conducted. It could be shown that wood extractives block the biorefinery solvent, thus leading to a lower capacity for the extraction of acetic acid (as shown in the graph). Additionally, their influence was investigated in single drop extractions. Using this method, it was shown for the first time that wood extractives also slow down the extraction of acetic acid by forming a barrier layer on the surface of solvent drops. In summary, wood extractives decrease both, the amount of acetic acid and the speed at which it is extracted.
Development of a solvent purification process
To restore the initial capacity of the solvent, a process for its continuous purification was searched. The concept for the removal of fatty acids was inspired by the human body. There, a substance called choline is used to transport fats and to prevent the accumulation of excess fat in the liver. Therefore, various chemically related substances called alkanolamines were screened for their capability to remove wood extractives out of the biorefinery solvent.
It was found that N-methylethanolamine (NMEA) and N,N-dimethylethanolamine (DMEA) enable the removal of over 99% of wood extractives. Furthermore, they simultaneously remove all solid particles and part of the furfural polymers from the biorefinery solvent. To remove residual amounts of NMEA or DMEA from the biorefinery solvent, the solvent needs to be washed with water. In this way, the biorefinery solvent can be easily purified and recycled back to the acetic acid extraction.
In order to provide an economically feasible process, the used alkanolamines (NMEA or DMEA) need to be separated from the impurities and reused in the purification process. Distillation trials were conducted and it could be shown that over 99% of NMEA or DMEA and process water could be recovered as distillate. The impurities (wood extractives, furfural polymers and particles) stay in the distillation sump and can be discharged.
Benefits for industrial biorefineries
The Wood K plus project was the first to demonstrate the negative effects of wood extractives contaminations in organic solvents on an industrial biorefinery. A solvent purification method was developed at lab scale and subsequently transferred to pilot scale. The patented process is capable of removing the undesirable impurities completely in an energy efficient way using fully recyclable chemicals. By purifying the biorefinery solvent, the acetic acid yield and plant availability can be increased. Due to the simultaneous removal of furfural polymers and particles, cleaning shutdowns and chemical costs can be reduced. In addition, the gained knowledge and the developed process can also be applied to other solvent extraction systems in the biorefinery industry.